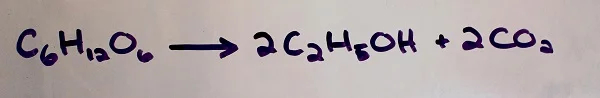Today we are going to tackle one of the most chemically precise areas in cooking: leavening. Whether you are using yeast or chemical leaveners, baking utilizes precise chemical reactions to give breads, cakes, and pastries their airy, light texture. Mess up the ratios and you can end up with flat, dense baked goods or “soapy”-tasting pastries.
For this reason, baking intimidates a lot of people. (Some of them are even chefs!) But don’t worry, the science is fairly straightforward, and we’re going to cover everything from yeast to baking powder so that you understand why and when you should use each leavening agent for perfect baked goods every time.
Leavening agents (chemical or otherwise) are those ingredients that react to produce the gases that inflate your dough and batters. Whether through enzymatic processes (yeast) or acid-base reactions (most chemical leaveners, like baking soda), leaveners give your baked goods the volume and texture that make them so appealing.
Yeast
Yeasts are a group of tiny single-celled fungi that have been making life tastier for at least 6,000 years. Though over a hundred different strains of yeast are known, one in particular – Saccharomyces cerevisiae – is the workhorse of brewers and bakers alike. Thought to have been originally isolated from the skin of grapes, it is employed daily to give us those magical products know as beer and bread (as well as a bunch of other amazingly delicious foods and beverages).
My body would not be what it is today without the help of Saccharomyces cerevisiae.
Yeasts metabolize or “eat” sugar for energy and emit carbon dioxide (the gas responsible for leavening bread) and alcohol (the product responsible for my drunk tweeting). Though both the CO2 and the ethanol are expelled when baked off in an oven (McGee 1984), they thankfully hang around in beer, giving it its trademark fizziness and booziness.
The conversion of sugar to alcohol and carbon dioxide that takes place in yeast cells is represented by the following equation:
What that means is that for every molecule of glucose sugar consumed by the yeast, two molecules of ethanol (drinking alcohol) and two molecules of carbon dioxide are produced.
Like any living organism, yeast has preferred living conditions. It’s most particular about temperature, and ideal ranges vary from strain to strain. Baker’s yeast produces the most gas at around 95F (McGee 1984), which is why it’s best to let your doughnuts rest in a warm environment.
This brief “nap” allows pastries like doughnuts to transform from boring flat circles like this:
To puffy, light, delicate delights such as this:
Want to see yeast in action? Follow this recipe by the Pioneer Woman for the best doughnuts of your entire life.
Though we have yeast to thank for many of the world’s wonders, it’s not very helpful when you’re working with a thin or weak batter. Yeast works on its own time, and takes around an hour to release that coveted carbon dioxide. This is fine for elastic, high-gluten products like bread and doughnuts, but runnier batters aren’t strong enough to hold gas bubbles for more than a few minutes, so a faster reaction is needed to leaven things like cakes, cookies, and muffins.
[Note: this is also why gluten-free goods can be so flat and dense; gluten helps hold the gases in, allowing your baked goods to properly inflate]
These faster reactions are accomplished through the use of chemical leaveners, most commonly baking soda and baking powder.
Baking Soda
Baking soda, which exists most commonly in the form of sodium bicarbonate, is a chemical leavener that utilizes acid-base reactions to yield carbon dioxide, which in turn puffs up your pastry.
Sodium Bicarbonate is a molecule that consists of one sodium ion (Na+) and one bicarbonate ion (HCO3-), but the bicarbonate is the important part. Once baking soda is exposed to water, the ions separate and the bicarbonate is free to react with whatever acid is present in the recipe. Common acids include chocolate, lemon juice, fermented dairy, coffee, and even brown sugar.
The most famous acid-base reaction involving baking soda is the ever popular vinegar and baking soda volcano, a rite of passage for a young, budding chemist.
Author's note: I'm sorry my handwriting is so terrible! For a more legible form of this equation, go here!
This reaction (in which the acid involved is acetic acid), or a reaction very similar to it, is what takes place when baking soda is exposed to an acidic environment. It’s simple, but crucial to achieving the right texture when baking thin-battered goods.
To see baking soda in action, whip up a batch of chocolate chip cookies. May I recommend Meg Hourihan’s completely impractical, but scientifically interesting recipe?
But what if your batter doesn’t contain any acidic ingredients?
That’s when baking powder comes in.
Baking Powder
Baking powder is a complete leavening system containing both the basic baking soda and crystalized acids. When added to liquid ingredients, the baking soda dissolves almost immediately and the acid goes into solution, allowing the two to react and produce the leavening gas. If the acid is highly soluble, it will go into solution quite quickly, reacting with the soda and releasing an initial set of bubbles (McGee 1984).
Other, less soluble acids will remain in their crystalized form for a while, sometimes until the cooking temperature is raised high enough to dissolve it.
The most common baking powders do both; they contain both very soluble and less soluble acids, which release gas upon first mixing and later again while baking.
Commercial bakers, who deal with large amounts of batter and dough on a daily basis, use baking powders with slow-release acids, so the gas doesn’t have time to escape before the batter makes it to the oven.
Because both baking soda and powder rely on precise chemical reactions, ratios are very important. This is why, unlike cooking, you can’t really “wing” a cake or a batch of brownies [Editor’s Note: Drunk baking says otherwise!]. Too little of a chemical leavener can result in flat, dense pastries; too much will result in poorly incorporated batters and bitter, soapy flavors. When mixed in proper ratios, both the acid and the base are used up in the reaction, and little excess is left behind.
My favorite way to utilize this powdered wonder is in the making of scones. At least I think they’re scones, they could be biscuits; I am still unclear on the difference.
It doesn’t matter; these are delicious. Somewhere in between a scone you would buy at a coffee shop and a biscuit you would get at Pine State or The Flying Biscuit, these are crumbly, moist, tender, and just a little sweet. These babies are truly versatile and work equally well at breakfast (with a little fresh goat cheese) or as dessert (with unsweetened whipped cream and macerated strawberries).
Claire’s Biscones
In a large bowl, cut 2.75 cups of all-purpose flour with 0.75 cups of chilled, cubed butter, using a pastry cutter or pulsing in the food processor until the mixture resembles very small rocks.
In a small bowl whisk together five tablespoons of sugar with five teaspoons of baking soda and half a teaspoon of kosher salt.
Mix the dry ingredients with the flour-butter mixture until just combined.
Create a well in the center and add half a cup of milk and one beaten egg. Combine until dough becomes a little sticky. (I used my hands.)
Place just slightly bigger than golf ball-sized mounds on a baking tray lined with parchment paper. Bake for 18 minutes at 400F, until edges just begin to brown.
Top with anything your little heart desires.
Isn’t science delicious?
Missed an installment of Savor the Science? You can read them all here. Got a food science question? Ask Claire in the comments section below!
Harold McGee. 1984. On Food and Cooking: The Science and Lore of the Kitchen. (New York: Scribner), 531-534.